-
Nu.Q® Technology
Our technology detects characteristic epigenetic changes in nucleosomes that occur from the earliest stages of cancer, sepsis and other diseases.
-
- Our Tests
- Human Health
-
Nu.Q® NETs
Nu.Q® NETs is a groundbreaking CE-marked diagnostic solution that clinicians can use to detect NETosis.
-
Nu.Q® Discover
Buy our Nu.Q® Discover H3.1 Research Use Only Assay
- Animal Health
-
Nu.Q® Vet Cancer Test
Nu.Q® Vet Cancer Test detects 76% of systemic cancers at 97% specificity.

Veterinarians.
Nu.Q® Vet Cancer Test
Earlier cancer detection improves outcomes.
It also improves the quality of life of the dog and its owner.
The Nu.Q® Vet Cancer Test is an affordable and accessible screening test to aid early detection. It is a simple, easy to use blood test for healthy, older dogs (7 years and older), as well as younger dogs (4 year and older) in breeds with an increased risk of developing cancer.
The Nu.Q® Vet Cancer Test is a science-backed tool that you can easily integrate into your preventive care program. It provides you with data-driven insights for a proactive care approach, to support your clinical decision making and enable you to guide pet owners with increased confidence.
How it works
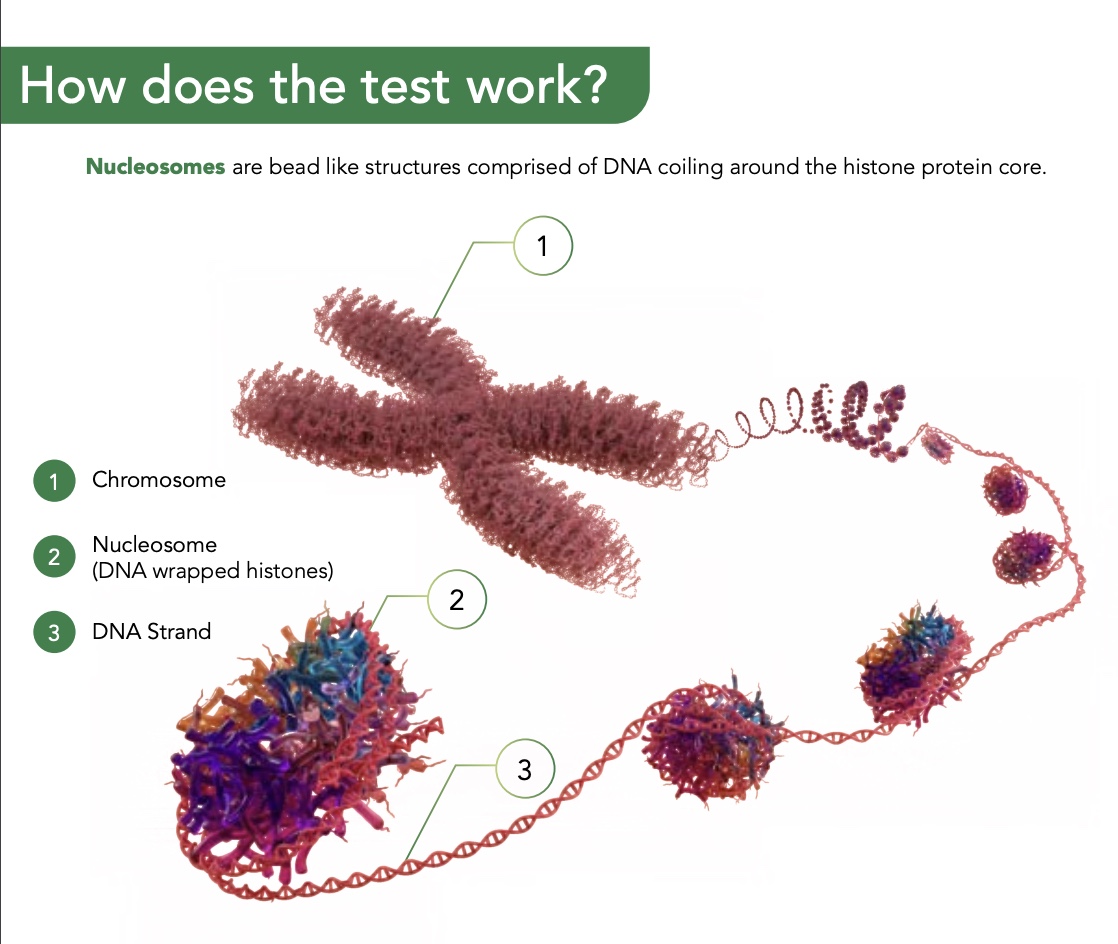
How does the Nu.Q® Vet Cancer Test work?
What does the Nu.Q® Vet Cancer Test measure?
When cancer is present, nucleosomes – originating from the cancer cells – enter the bloodstream. They can be captured using antibodies tailored specifically to detect nucleosomes.
The Nu.Q® Vet Cancer Test quantifies these circulating nucleosome levels in the blood. The test needs just 0.5ml of plasma, obtainable from a 2-5ml blood draw after centrifugation.
By measuring and analyzing nucleosomes, our Nu.Q® Vet Cancer Test can help identify patients who may have a cancer. This must then be confirmed by follow-up procedures – for example, a biopsy or scan.
What types of cancer has the Nu.Q® Vet Cancer Test been able to detect?
The Nu.Q® Vet Cancer Test can detect cancers such as lymphoma and hemangiosarcoma, even at early stages. Preliminary data suggests that this screening test can also detect some instances of Mast Cell tumours, malignant melanomas and Histiocytic Sarcoma. The current Nu.Q® Vet Cancer Test more reliably detects systemic cancers rather than soft tissue or localized cancers.

How to take a sample and interpret the results
Visit our Nu.Q® Vet Cancer Test product information page for a step-by-step guide on how to take a sample from your patient and interpret the results.

The research behind the results
In a peer-reviewed and published case series, the Nu.Q® Vet Cancer Test was shown to detect 76% of systemic cancers.
- 77% lymphoma
- 82% hemangiosarcoma
- 54% histiocytic sarcoma
Our test was able to identify approximately 50% of all cancers researched at 97% specificity.
Lymphoma is the most common form of canine cancer and together with hemangiosarcoma make up approximately one-third of all cancers.
Study details:
- A total of 662 dogs were included in the study (134 healthy and 528 with cancer).
- A variety of breeds, weights and cancer stages were represented
- 7 cancers were evaluated: Lymphoma, Malignant melanoma, Hemangiosarcoma, Mast cell tumors, Osteosarcoma, Histiocytic sarcoma and Soft tissue sarcoma.
of systematic cancers detected
of all cancers researched
Introduce Nu.Q® Vet into your clinic
Our test is currently available in the United States, Europe, and Asia through reference laboratories which include IDEXX and Heska/Antech. It is also available as an in-house test exclusively via Heska/Antech.
You can find details of your local distributor below.
OUR DISTRIBUTOR NETWORK

Heska/Antech
Nu.Q® Canine Cancer Test
(U.S.)
Test code: 313100

IDEXX
Nu.Q® Canine Cancer Screen
(U.S.)
Test code: 8993

NationWide
Nu.Q® Vet Cancer Test
(UK)

Veterinary Pathology Group (VPG)
Nu.Q® Vet Cancer Test
(UK & IRL)

DNA Tech
Nu.Q® Vet Cancer Test
(Portugal)

scil animal care company
Nu.Q® Vet Cancer Test
(Italy)

Fujifilm Vet Systems Co. Ltd
Nu.Q® Vet Cancer Test
(Japan)

Sage Healthcare
Nu.Q® Vet Cancer Test
(Singapore)

Vita Genomics
Nu.Q® Vet Cancer Test
(Taiwan)
Brochure & Clinical Papers
Next steps
Get in touch with our vet team
Our expert team will be on hand to offer guidance and support and to fulfil your needs.
"*" indicates required fields
Contact Nu.Q® Vet Team
Modal Contact Form (Vet - Veterinarians) - Pardot
"*" indicates required fields